Have you ever wondered how scientists can tell whether a medicine is pure, if a food contains hidden additives, or whether a chemical reaction has actually finished? One of the simplest yet most powerful answers is Thin Layer Chromatography (TLC).
Chromatography is the science of separating mixtures, and TLC is its most accessible form—fast, inexpensive, and easy to perform in any basic laboratory. For students, it’s often the very first hands-on analytical technique they learn. Unlike complex machines such as HPLC or GC, thin layer chromatography uses a coated plate (stationary phase) and a moving solvent (mobile phase) to separate compounds based on their polarity and interactions. By calculating the Rf value and observing the spots under UV light or using simple chemical stains, even beginners can identify, compare, and analyze compounds effectively.
In this article, we’ll explore the definition, classification, nomenclature, examples in daily life, and importance of thin layer chromatography—everything you need to build a strong conceptual foundation and score high in your coursework. Whether you’re preparing for a lab practical, revising for exams, or simply curious about how TLC connects to pharmaceuticals, food safety, and everyday applications, this guide will give you a clear, student-friendly roadmap.
What is Chromatography?
At its core, chromatography is a separation technique used to divide a mixture into its individual components. The word comes from the Greek “chroma” (color) and “graphein” (to write), reflecting the colorful separations first observed when pigments moved across a medium. In simple terms, it is a method that relies on the different affinities of molecules toward two phases: a stationary phase (which stays fixed) and a mobile phase (which moves through the stationary medium).
For students, understanding chromatography basics is essential, as it forms the backbone of many analytical techniques in chemistry, biochemistry, and biology. Whether separating plant pigments in a classroom experiment or testing for drug purity in a pharmaceutical lab, the principle remains the same—molecules travel at different speeds, leading to clear separation. This makes chromatography one of the most widely used analytical tools in science.
Thin Layer Chromatography (TLC)
Among the different types of chromatography, Thin Layer Chromatography (TLC) is one of the simplest and most widely applied methods. By definition, TLC is a planar chromatography technique in which a thin layer of adsorbent material, such as silica gel or alumina, is coated on a plate (the stationary phase), and a liquid solvent (the mobile phase) moves across it, separating compounds based on their polarity and interactions.
The separation is often measured using the Rf value (retention factor), which provides a quick way to identify and compare compounds. Because TLC is inexpensive, easy to perform, and requires minimal equipment, it is a favorite choice for teaching laboratories, research experiments, and industrial quality checks.
Role of TLC in Science and Industry
Though simple, TLC has a wide range of applications that make it indispensable in modern science. In chemical laboratories, it is routinely used for reaction monitoring, allowing researchers to check whether a reaction is complete within minutes. The pharmaceutical industry, TLC plays a vital role in purity checks and preliminary analytical techniques before more advanced methods like HPLC are employed. Biological sciences, it helps identify natural products such as amino acids, lipids, and plant alkaloids, contributing to both academic research and industrial applications.
For students, learning TLC is not just about passing exams—it is about mastering a technique that connects theory with real-world problem-solving. Its simplicity, speed, and versatility make it an essential analytical method across disciplines.
Definition of Thin Layer Chromatography (TLC)
Formal Definition
Thin Layer Chromatography (TLC) is a widely used TLC method of planar chromatography, where compounds are separated on a flat surface coated with a thin layer of adsorbent material. In this technique, the stationary phase is usually silica gel or alumina spread evenly on a plate made of glass, plastic, or aluminum. The mobile phase is a liquid solvent or a mixture of solvents that moves across the plate by capillary action.
In simple terms, TLC is a separation technique based on differences in polarity and adsorption of compounds on a thin layer of solid material. It is cost-effective, rapid, and easy to perform, making it an essential analytical tool for students and researchers.
Key Components of TLC
- Stationary Phase
- A thin coating of silica gel (polar adsorbent) or alumina applied on a plate.
- This layer acts as the surface on which compounds are adsorbed and separated.
- Sometimes plates are treated with fluorescent indicators (e.g., F254) to help visualize compounds under UV light.
- Mobile Phase (Solvent System)
- The solvent or solvent mixture that moves up the plate due to capillary action.
- Common examples: hexane–ethyl acetate, dichloromethane–methanol, or water-based systems depending on the polarity of compounds.
- Choosing the right solvent system is crucial for achieving clear separation.
Principle of Separation in TLC
The principle of Thin Layer Chromatography lies in adsorption chromatography. Different compounds in a mixture have varying affinities (attractions) toward the stationary phase and the mobile phase.
- Polar compounds are strongly attracted to the polar stationary phase (silica gel or alumina) and move slowly.
- Non-polar compounds prefer the mobile phase (solvent) and travel faster.
- This difference in movement results in the separation of compounds along the plate.
The process is aided by capillary action, where the solvent naturally rises up the plate, carrying the sample molecules along with it.
Visual Elements in TLC
To analyze a TLC experiment, students must be familiar with its visual markers:
- Baseline – the starting line where the sample is spotted before developing.
- Solvent Front – the highest point reached by the mobile phase during the run.
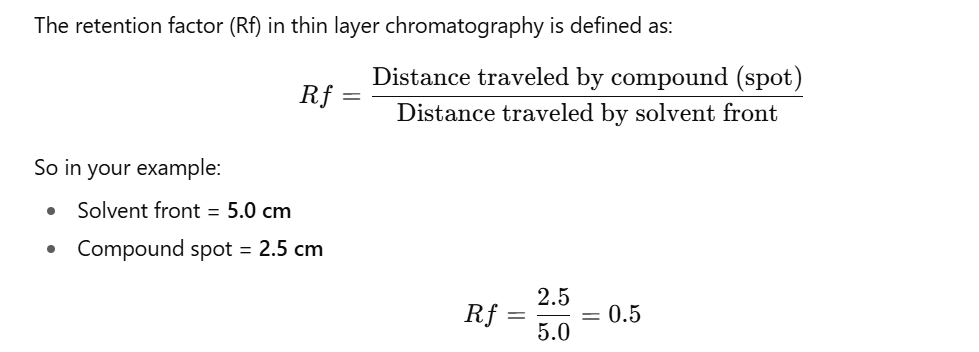
- Rf Value (Retention Factor) – a numerical value that indicates how far a compound traveled relative to the solvent front.
- Example: If the solvent front moved 5.0 cm and the compound spot moved 2.5 cm, the Rf = 0.5.
- The Rf value helps in identifying compounds by comparing them with known standards.
Why this matters for students:
Understanding the definition, key components, principle of separation, and visual elements of TLC forms the foundation for exam answers and laboratory skills. It connects theory with practice, helping you not only describe TLC in words but also perform and interpret it during lab practicals.
Classification of Chromatography (with Focus on TLC)
Chromatography is not a single technique but a family of separation methods that differ based on the type of interaction between the sample molecules and the stationary phase. For students, knowing the classification of chromatography is crucial for both theory exams and lab applications.
Broad Classification of Chromatography
- Adsorption Chromatography
- This method relies on the adsorption of molecules onto the surface of a solid stationary phase.
- Thin Layer Chromatography (TLC) and Column Chromatography are the best-known examples.
- Compounds separate because different molecules interact differently with the adsorbent (silica gel or alumina).
- Partition Chromatography
- Based on the distribution (partitioning) of molecules between two immiscible phases: one stationary and one mobile.
- Paper Chromatography and High-Performance Liquid Chromatography (HPLC) often use this principle.
- Ion-Exchange Chromatography
- Used for separating charged particles (ions) based on attraction to an oppositely charged stationary phase.
- Widely used in biochemistry to separate amino acids, proteins, and nucleotides.
- Affinity Chromatography
- Highly specific method where molecules are separated based on biological interactions (e.g., enzyme–substrate, antigen–antibody).
- Common in biological sciences and research labs for purifying proteins or DNA.
Placement of Thin Layer Chromatography (TLC) in This Classification
- Thin Layer Chromatography (TLC) is classified under adsorption chromatography.
- It is a form of planar chromatography, since separation occurs on a flat stationary phase (plate) rather than in a column.
- Compared to paper chromatography, which also uses a planar surface, TLC provides better resolution, faster results, and a wider range of visualization methods.
- Thus, in the classification system, TLC stands as a simple, powerful, and widely used planar adsorption chromatography technique.
Analytical TLC vs Preparative TLC
TLC can be further divided into two practical categories based on its purpose:
- Analytical TLC
- The most common form taught in undergraduate labs.
- Used to analyze mixtures, calculate Rf values, and monitor reaction progress.
- Requires very small amounts of sample.
- Provides qualitative information rather than exact quantities.
- Preparative TLC
- Used when larger amounts of compound need to be separated and collected.
- Involves spotting more sample on a larger TLC plate and then physically scraping off the separated bands for recovery.
- Provides quantitative information and actual compound isolation.
- More common in research and industrial labs than in student practicals.
Key takeaway for students:
Chromatography can be divided into several categories, but Thin Layer Chromatography (TLC) belongs to adsorption chromatography and is a type of planar chromatography. Understanding its placement in this classification, and the difference between analytical TLC and preparative TLC, gives you a complete exam-ready answer while also strengthening your practical knowledge for laboratory work.
Nomenclature and Key Terms in Thin Layer Chromatography (TLC)
When studying Thin Layer Chromatography (TLC), it is important to understand the basic terminology and nomenclature used in laboratory manuals, textbooks, and exam questions. Below is a clear explanation of the most essential TLC key terms, written in a glossary style for easy learning.
1. Rf Value (Retention Factor)
- Definition: The Rf value, or retention factor, is the ratio that describes how far a compound has traveled on the TLC plate compared to the solvent front.
- Importance: The Rf value is used for compound identification, comparison with known standards, and monitoring reaction progress.
2. Stationary Phase
- The stationary phase is the solid surface that remains fixed during chromatography.
- In TLC, this is typically:
- Silica gel (most common, polar adsorbent)
- Alumina (used for less polar compounds)
- Reverse-phase TLC plates (RP-18) coated with modified silica, where the surface is non-polar and the mobile phase is relatively polar.
- The stationary phase plays a critical role in separation, as compounds adsorb differently depending on their polarity.
3. Mobile Phase (Eluent)
- The mobile phase, also called the eluent, is the solvent or mixture of solvents that moves up the TLC plate by capillary action.
- Common solvent systems include:
- Hexane/ethyl acetate (widely used for organic compounds)
- Dichloromethane (DCM)/methanol
- Toluene, acetone, chloroform, or mixtures depending on the compound’s polarity.
- The choice of mobile phase directly affects the separation quality and the Rf values obtained.
4. Visualization Methods
Most compounds are not visible to the naked eye after running TLC. Therefore, TLC visualization techniques are used to detect and analyze separated spots.
- UV Light (UV 254 nm):
Many TLC plates are coated with fluorescent indicators (F254). Under UV light, compounds appear as dark spots against a glowing background. - Iodine Chamber:
Exposing TLC plates to iodine vapor makes organic compounds appear as brown spots. This method is quick and non-destructive. - Chemical Stains (TLC stains):
Spraying or dipping plates in chemical reagents produces colored spots for specific compound classes:- Anisaldehyde stain – for sugars, steroids, terpenes.
- Ninhydrin stain – for amino acids, peptides, proteins (purple/blue spots).
- Vanillin stain – for alcohols, phenols, and lipids.
- Phosphomolybdic acid (PMA), potassium permanganate, sulfuric acid stains – general detection of many organic compounds.
Visualization is one of TLC’s strengths because multiple methods allow flexibility in detecting different types of molecules.
Key Takeaway for Students:
By mastering these terms — Rf value, stationary phase, mobile phase, and visualization methods — you’ll be able to confidently describe TLC in exams, understand lab instructions, and correctly interpret TLC plates in practicals.
Examples of TLC in Daily Life & Laboratory Applications
Thin Layer Chromatography (TLC) is more than just a laboratory technique you read about in textbooks — it’s widely used in real-world applications across pharmaceuticals, food science, biochemistry, and academic labs. By understanding how TLC works in these fields, you’ll not only strengthen your exam preparation but also appreciate its practical importance.
1. Pharmaceuticals – Checking Purity of Drugs
In the pharmaceutical industry, TLC plays a vital role in ensuring that medicines are safe and effective. One of the most common uses is TLC in pharmaceutical analysis, where it helps in:
- Purity testing: TLC quickly identifies whether a drug contains impurities or not.
- Pharma ID tests: By comparing the TLC pattern of a sample with that of a standard drug, pharmacists can confirm the identity of active pharmaceutical ingredients (APIs).
- Detecting adulteration: Low-quality or counterfeit medicines can be spotted using TLC, which is crucial for patient safety.
2. Food Industry – Testing for Preservatives, Colors & Adulterants
Food safety is a major concern, and TLC in food analysis is widely used to check what really goes into the food we eat. Examples include:
- Detecting artificial food colors and preservatives in packed foods.
- Identifying oil adulteration, such as mixing cheaper oils into pure ones.
- Monitoring the presence of toxic chemicals or pesticide residues in fruits and vegetables.
Students should note that TLC is often preferred here because it’s a fast and low-cost screening method, especially useful when analyzing large numbers of food samples.
3. Biochemistry Labs – Separation of Biomolecules
In the field of biochemistry, TLC is a go-to technique for analyzing complex biological samples. Some common applications are:
- TLC of amino acids: Helps separate and identify amino acids in protein hydrolysates.
- TLC of lipids: Used to study fat composition in biological samples.
- TLC of natural products: Widely applied to detect alkaloids, flavonoids, and plant metabolites in herbal extracts.
In exams, you can highlight that TLC is useful for qualitative studies in natural product research, especially when combined with visualization techniques like ninhydrin spray for amino acids.
4. Academic Chemistry Labs – Monitoring Reactions
For chemistry students, the most common exposure to TLC is in the academic laboratory. Here, it is frequently used for:
- TLC in reaction monitoring: Students check if a chemical reaction has completed by comparing the TLC spots of reactants and products.
- Co-spotting techniques: By spotting both the sample and reference side by side, students confirm product identity with greater accuracy.
For practical exams, always remember that TLC is a quick checkpoint during organic synthesis experiments.
Key Takeaway for Students:
Thin Layer Chromatography (TLC) is not just a theoretical concept — it is a powerful analytical tool with wide applications. Whether it’s TLC in pharmaceutical analysis, TLC in food analysis, TLC of amino acids and lipids, or TLC in reaction monitoring, its relevance extends from the classroom to real-world industries.
Importance of Thin Layer Chromatography
Thin Layer Chromatography (TLC) is one of the most versatile and widely used separation techniques in chemistry and life sciences. Its importance spans across education, research, and industry, making it a fundamental concept for students and professionals alike. Understanding the applications and advantages of TLC will help you not only in exams but also in appreciating how this technique connects theory to real-world use.
1. Educational Importance of TLC for Students
For undergraduate chemistry and biochemistry students, TLC is an essential learning tool because it is:
- Simple and visual: Students can see the separation of compounds as distinct spots on a TLC plate, making it easy to grasp.
- Cost-effective: Unlike high-end instruments, TLC requires minimal setup, making it ideal for academic laboratories.
- Hands-on practice: Students learn concepts of polarity, solvent systems, and compound identification in a direct, visual manner.
For your graduation exams, remember that TLC is often introduced in labs because it combines theory with simple practical skills—a foundation for advanced techniques later.
2. Research Significance of TLC
In research, TLC serves as a fast and reliable preliminary screening method before moving to advanced instruments. Some major applications include:
- Qualitative analysis: Detecting the presence or absence of a compound in a mixture.
- Reaction progress monitoring: TLC quickly shows if a chemical reaction is complete, making it a favorite in organic synthesis.
- Purity check: By comparing a sample with a standard, researchers confirm whether a compound is pure or contaminated.
- Method development: TLC helps optimize solvent systems before applying costly methods like HPLC.
When writing exams, emphasize that TLC is valued in research because it saves both time and resources while giving rapid insights.
3. Industrial Importance of TLC
Beyond academia, TLC applications extend into many industries:
- Pharmaceuticals: Used for drug identity tests and impurity detection (TLC in pharmaceutical analysis).
- Food safety: Helps detect preservatives, colorants, and adulterants (TLC in food analysis).
- Cosmetics: Ensures product purity and checks for harmful additives.
- Herbal medicine & natural products: TLC is widely used to authenticate plant extracts and identify bioactive compounds (TLC in biochemical analysis).
Students should note that industries value TLC because it is inexpensive, quick, and reliable for quality control and safety checks.
4. Comparison with Other Methods
While TLC is important, it’s often compared with other chromatographic techniques:
- TLC vs HPLC:
- TLC is inexpensive and fast, ideal for preliminary analysis.
- HPLC (High-Performance Liquid Chromatography) is more advanced, offering precise quantitative results but at a higher cost.
- Exam tip: TLC is often called a qualitative tool, while HPLC is a quantitative tool.
- TLC vs Column Chromatography:
- TLC is quicker, requires less solvent, and is mainly used for analytical purposes.
- Column chromatography is better for large-scale separations but takes longer.
- Exam tip: TLC is best for small samples, while column chromatography suits preparative work.
Key Takeaway for Students:
Thin Layer Chromatography (TLC) is important at multiple levels:
- Educational (simple, cost-effective learning tool),
- Research (qualitative analysis, reaction monitoring, purity checks), and
- Industrial (pharmaceuticals, food, cosmetics, herbal medicine).
When preparing for exams, always highlight that TLC is a foundation technique—simple yet powerful, and often the first step before moving to advanced chromatographic methods.
Conclusion
As we reach the end of this discussion, let’s quickly recap the journey of Thin Layer Chromatography (TLC) and why it holds such a central place in analytical chemistry.
We began with the definition of TLC, understanding it as a simple and efficient separation technique. Then we moved into its classification and nomenclature, which helped clarify how TLC is structured and named. With real-world examples of TLC applications—from pharmaceuticals and food testing to biochemistry and academic labs—you saw how this technique is not just theoretical but also widely practical. Finally, we explored its importance across education, research, and industry, reinforcing why TLC is often the first analytical method introduced to chemistry students.
At its core, Thin Layer Chromatography (TLC) is a fundamental analytical technique because it is:
- Easy to use and highly visual, making it perfect for BSc-level learning.
- Versatile, applied in pharmaceuticals, food safety, herbal medicine, and biochemical analysis.
- Cost-effective and reliable, often serving as a first step before advanced methods like HPLC.
For students preparing for graduation exams, TLC is more than just a practical experiment in the lab. It is a foundation for understanding advanced analytical techniques that you will encounter in higher studies and professional research.
Key takeaway: Mastering Thin Layer Chromatography will not only help you score well in exams but also give you a solid grounding for your future in chemistry, biochemistry, and pharmaceutical sciences.
So, as you revise, keep TLC in mind not just as a chapter in your syllabus, but as a gateway technique that bridges classroom knowledge with real-world applications.
Frequently Asked Questions
1. What is Thin Layer Chromatography (TLC) in simple words?
Thin Layer Chromatography (TLC) is a simple test that helps separate and identify small parts of a mixture. It is used in science labs, food testing, and medicine to check if something is pure or mixed with other substances.
2. Why is TLC important for students?
TLC is important for BSc students because it is easy to perform, low-cost, and very visual. It helps them understand how mixtures can be separated and how to check the purity of samples.
3. How is TLC used in the pharmaceutical industry?
In the pharmaceutical industry, TLC is used to check the purity of drugs, identify medicines, and detect adulteration. This ensures that patients get safe and effective medicines.
4. How does TLC help in the food industry?
TLC in food analysis helps detect artificial colors, preservatives, adulterants, and harmful chemicals. This is important for keeping food safe for families and children.
5. What are some examples of TLC in daily life?
Examples include:
- Checking if a drug is pure in pharmacies.
- Testing food for harmful additives.
- Studying amino acids, lipids, and natural products in labs.
- Monitoring chemical reactions in student experiments.
6. What is the difference between TLC and HPLC?
- TLC is simple, quick, and cost-effective, mainly for qualitative analysis (to see what is present).
- HPLC is advanced, expensive, and used for quantitative analysis (to measure how much is present).
7. What is the difference between TLC and column chromatography?
- TLC is fast, cheap, and needs only a small sample.
- Column chromatography is slower but can separate large amounts of material.
8. Why do researchers use TLC before advanced tests?
Researchers use TLC because it is quick and inexpensive. It helps with reaction monitoring, purity check, and method development before moving on to costly methods like HPLC.
9. Is TLC used in herbal medicine?
Yes. TLC is widely used to check natural products, herbal extracts, and plant-based medicines to ensure they are genuine and safe.
10. Why should BSc students learn TLC?
BSc students should learn TLC because it is a foundation technique in chemistry. It builds the base for advanced studies and helps them perform well in practical exams, research projects, and industry jobs.
About the Author – Gnanasree Vankadari
Gnanasree Vankadari, M.Sc. Biotechnology (Gold Medalist), is a researcher and science communicator with a strong academic background in microbiology and biotechnology. She has presented award-winning research on cryogenics and conducted a postgraduate project on Bacillus calusii. At Biology Handbook, she shares her passion for life sciences by simplifying complex biological concepts for students and readers around the world.