Chromatography is one of those techniques that every B.Sc. student encounters in practical labs, viva questions, and competitive exams. Among the many forms of chromatography, paper chromatography stands out as the simplest, most affordable, and most student-friendly method to understand how scientists separate and analyze mixtures. Whether you’re studying the principle of paper chromatography, setting up the instrumentation with Whatman filter paper and a solvent system, or exploring its applications in separating amino acids, plant pigments, food dyes, and even forensic samples, this technique is both exam-relevant and practically useful.
In this guide, we’ll break down the principle, instrumentation, and applications of paper chromatography step by step — with easy explanations, real-life examples, and answers to common student questions like “Why is pencil used instead of ink?” or “How do you calculate Rf value?”. By the end, you’ll not only understand the theory but also know how to apply it in your lab experiments, reports, and exam answers.
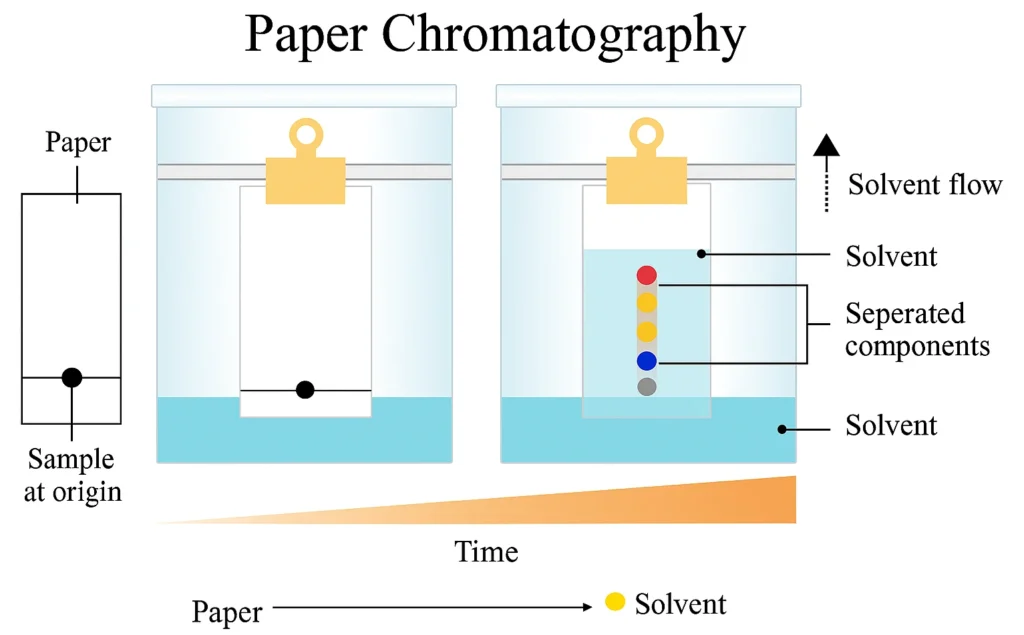
Principle of Paper Chromatography
What is the Principle of Paper Chromatography?
The principle of paper chromatography is based on the idea that different compounds in a mixture will move at different speeds when carried by a solvent through a piece of paper. This happens because each substance has a different affinity (or attraction) towards the two phases involved:
- Stationary phase: In paper chromatography, the stationary phase is not just the paper itself but the thin film of water molecules trapped in the cellulose fibers of Whatman filter paper. Substances that are more soluble in this stationary water layer tend to stay near the starting point.
- Mobile phase: The mobile phase is a solvent system (such as butanol, acetic acid, and water) that moves through the paper. Compounds that dissolve better in this solvent travel further up the paper.
The movement is possible because of capillary action—the natural tendency of liquids to rise through narrow spaces without external force. As the solvent front moves upward, it carries the components of the mixture along with it, separating them based on how strongly they interact with the stationary vs. the mobile phase.
In simple words: substances that “like” the solvent move faster, while those that “like” the paper move slower. This difference is what gives us the separation pattern, or chromatogram.
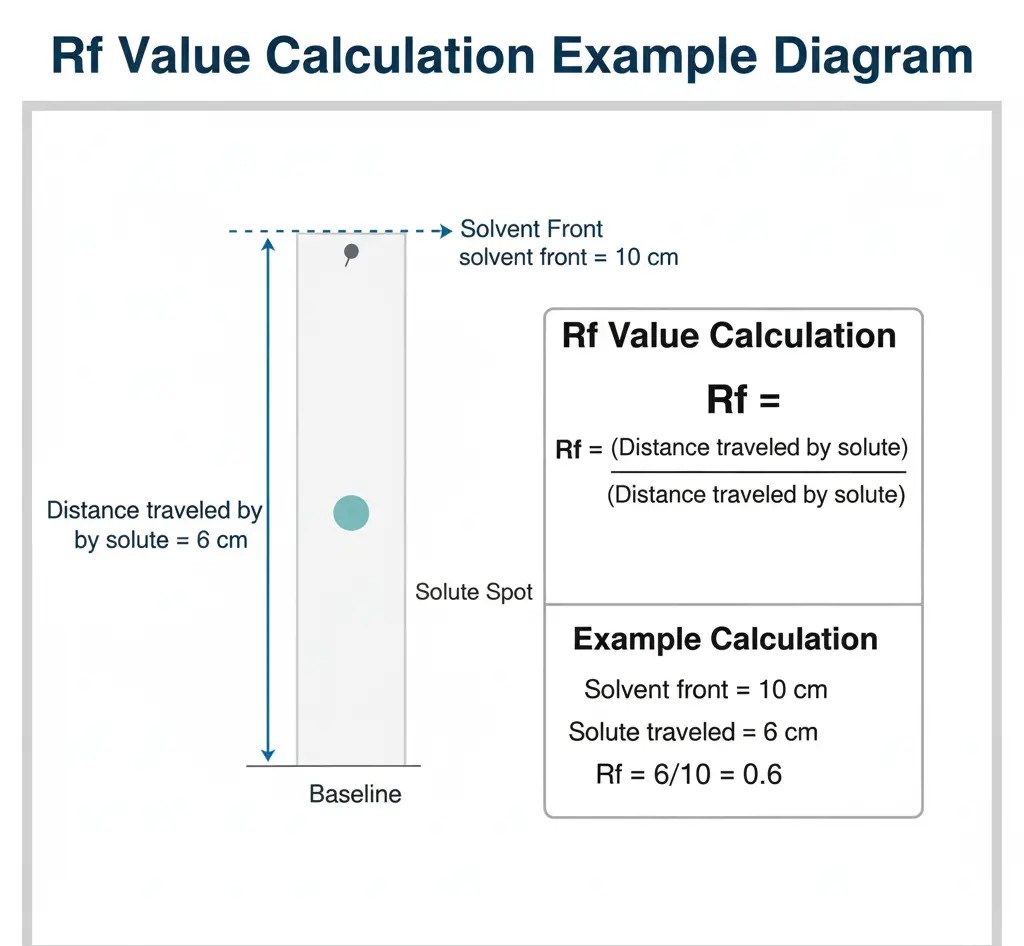
Rf Value in Paper Chromatography
To make chromatography results meaningful, we calculate something called the Retention factor (Rf value). It helps identify compounds and compare results between experiments.
- Definition: The Rf value in paper chromatography is the ratio of the distance traveled by the solute (the spot) to the distance traveled by the solvent front.
- Formula:
Rf=Distance traveled by solute (spot)Distance traveled by solvent frontRf = \frac{\text{Distance traveled by solute (spot)}}{\text{Distance traveled by solvent front}}Rf=Distance traveled by solvent frontDistance traveled by solute (spot) - Example: If the solvent front moves 10 cm and an amino acid spot moves 6 cm, then:
Rf=610=0.6Rf = \frac{6}{10} = 0.6Rf=106=0.6
Factors affecting Rf value:
- Solvent polarity – Polar solvents carry polar compounds further.
- Type of paper – Different grades of Whatman paper hold water differently.
- Temperature and humidity – Higher temperature can change solubility and mobility.
Remember: Rf values are unitless, because it’s a ratio of two distances.
Understanding how to calculate Rf with an example is essential for exams, as many question papers ask students to work out values from experimental data.
Adsorption vs. Partition in Paper Chromatography
Students often wonder: Is paper chromatography based on adsorption or partition? The answer is—mainly partition chromatography.
- The process depends on how compounds distribute themselves between the stationary water phase in the paper and the mobile solvent phase. This is explained by the partition coefficient, which measures how a substance divides between two phases.
- However, in some cases, adsorption also plays a minor role. Compounds can temporarily stick to the surface of cellulose fibers through hydrogen bonding or van der Waals forces.
So, while paper chromatography is generally classified as partition chromatography, it is best to remember that both partition and adsorption can contribute to the separation process.
Instrumentation of Paper Chromatography
To perform paper chromatography, only a few basic instruments and materials are required. This simplicity is what makes it one of the most popular chromatography on paper techniques for teaching, learning, and laboratory practice. Each part of the setup plays a crucial role in ensuring accurate separation and reliable results. Let’s go step by step.
Chromatography Paper (Stationary Phase)
The heart of this experiment is the chromatography paper, which serves as the stationary phase in paper chromatography. Commonly, Whatman filter paper No.1 is used because it has uniform texture and good capillary properties.
The paper itself doesn’t just act as a physical support—it contains cellulose fibers that hold a thin layer of water molecules. This water layer forms the actual stationary phase where the solutes get distributed during the experiment. Substances that have more affinity for the stationary water phase remain closer to the baseline, while those more soluble in the solvent system travel further up.
Mobile Phase / Solvent System
The second essential component is the mobile phase, also called the solvent system. The solvent moves through the paper by capillary action and carries the analytes with it. Choosing the right solvent system is critical because it directly affects the Rf value in paper chromatography.
- A common solvent mixture used for separating amino acids is butanol:acetic acid:water (BAW).
- The polarity of the solvent determines how far a compound travels: polar solvents move polar molecules further, while non-polar solvents are better for non-polar compounds.
Sample Spotting and Baseline
Before starting, the sample solution is carefully applied as a small spot near the bottom of the chromatography paper, on a line called the baseline or origin line.
- The baseline is always drawn with a pencil, never with ink, because pencil marks do not dissolve in the solvent. Ink, on the other hand, could spread and interfere with the chromatogram.
- The sample is usually applied using a spotting capillary or micropipette to ensure a small, concentrated spot.
- Proper sample preparation in paper chromatography is important for achieving sharp, well-defined spots.
Developing Chamber
Once the paper is prepared, it is placed inside a developing chamber, which is usually a closed container containing a small amount of solvent.
- The chamber is saturated with solvent vapors, which improves consistency in the movement of the solvent front.
- Depending on the method, paper chromatography can be performed in different modes:
- Ascending chromatography – solvent rises upward by capillary action.
- Descending chromatography – solvent moves downward with the help of gravity.
- Radial (circular) paper chromatography – solvent moves outward in circles from a central spot.
- Two-dimensional (2D) chromatography – the paper is developed in one solvent system, dried, then developed again at a right angle in another solvent system.
Visualization Methods
Once the chromatogram is developed, the separated spots often need to be made visible. This process is called visualization in paper chromatography.
- For amino acids, the most common method is spraying the chromatogram with ninhydrin reagent, which produces purple or blue-colored spots where amino acids are present.
- For organic compounds like lipids or sugars, an iodine chamber or UV light source can be used to visualize otherwise invisible spots.
Visualization not only confirms the presence of compounds but also allows for accurate Rf value calculations.
Applications of Paper Chromatography
One of the biggest strengths of paper chromatography is its wide range of applications across education, research, and industry. For students, this technique is not only important for lab experiments but also a frequent topic in theory exams and viva questions. Below are some of the most common and practical applications.
Separation of Amino Acids and Proteins
One classic use of paper chromatography is in the separation of amino acids. When a mixture of amino acids is spotted on chromatography paper and developed in a suitable solvent system, each amino acid travels a different distance depending on its solubility and polarity.
To visualize the separated spots, the paper is sprayed with ninhydrin reagent. Ninhydrin reacts with amino acids to produce colored spots (usually purple or blue), making them easy to detect. This method is widely used in teaching labs for detecting amino acids by paper chromatography and in biochemistry for studying proteins and peptides.
Plant Pigment Separation
A popular experiment for students is the paper chromatography of plant pigments. For example, pigments can be extracted from spinach leaves and separated into different bands such as chlorophyll a, chlorophyll b, carotenoids, and xanthophylls.
This simple experiment for students demonstrates how even complex natural mixtures can be broken down into individual components. It’s an effective way to understand concepts like solubility, polarity, and Rf values while connecting theory to a real-life biological system.
Food Industry Applications
In the food industry, paper chromatography is used for identifying and testing food dyes, colorants, and preservatives. Many food items contain mixtures of synthetic and natural colors that can be separated and analyzed on paper.
This technique can also be used in quantitative paper chromatography, where the intensity of the spot correlates with the concentration of the substance. For students, this highlights how simple classroom techniques are applied in large-scale industries to maintain food safety and quality.
Forensic Applications
One of the most fascinating uses is in forensic applications of paper chromatography. In criminal investigations, this method helps in ink separation and the analysis of dyes from documents.
For example, if two suspects use different pens to sign a document, paper chromatography can reveal differences in ink composition, helping experts detect forgeries. Its simplicity and low cost make it a valuable tool in crime labs, especially for preliminary testing.
Pharmaceutical and Clinical Applications
In the pharmaceutical field, paper chromatography in pharmaceuticals is applied to test the purity of drugs and detect the presence of impurities. This ensures that medicines meet quality standards before reaching patients.
In clinical biochemistry applications, it is used to identify metabolites in biological fluids such as urine and plasma. This can assist in diagnosing metabolic disorders and monitoring treatment outcomes.
Advantages and Limitations
Like any scientific method, paper chromatography has its strengths and weaknesses.
Advantages of paper chromatography:
- It is cheap, simple, and easy to perform, making it ideal for classroom teaching and small labs.
- Requires minimal equipment and small sample sizes.
- Useful for quick, qualitative analysis.
Limitations of paper chromatography:
- It offers lower resolution compared to advanced methods like Thin Layer Chromatography (TLC) or High-Performance Liquid Chromatography (HPLC).
- Not suitable for separating highly complex mixtures or compounds that are very similar in polarity.
For exams, students are often asked to compare paper chromatography vs TLC. While both are based on similar principles, TLC provides sharper separations and can handle more complex samples, whereas paper chromatography remains a foundational, educational technique.
Conclusion
Paper chromatography remains one of the most valuable and accessible separation techniques for students and researchers. Its principle lies in the distribution of compounds between a stationary phase (water held in the cellulose fibers of paper) and a mobile phase (the solvent system). With simple instrumentation—chromatography paper, solvent system, spotting capillary, and a developing chamber—students can separate mixtures effectively and calculate Rf values to identify compounds.
From amino acids and plant pigments to food dyes, pharmaceuticals, and forensic inks, the applications of paper chromatography prove just how versatile this method is. While it may not match the precision of TLC or HPLC, it is still highly valued in education and basic research due to its low cost, simplicity, and reliability.
For B.Sc. chemistry students, mastering this topic means being well-prepared for theory exams, viva questions, and lab practicals. Paper chromatography not only explains core principles of separation but also connects science directly to real-world uses in medicine, food, and forensic science.
Quick Revision Summary (Exam Notes)
- Principle: Separation occurs due to differences in partitioning of compounds between the stationary phase (water in paper) and mobile phase (solvent).
- Stationary Phase: Water molecules bound to Whatman filter paper (cellulose fibers).
- Mobile Phase: Solvent system, e.g., butanol:acetic acid:water.
- Rf Value:
Rf=Distance traveled by soluteDistance traveled by solvent frontRf = \frac{\text{Distance traveled by solute}}{\text{Distance traveled by solvent front}}Rf=Distance traveled by solvent frontDistance traveled by solute- Unitless, affected by solvent polarity, type of paper, and temperature.
- Instrumentation: Paper, solvent system, baseline/origin line (marked with pencil), spotting capillary, developing chamber, visualization reagents (ninhydrin, iodine, UV light).
- Applications:
- Amino acids separation (ninhydrin spray).
- Plant pigment separation (chlorophyll, carotenoids).
- Food color and preservative testing.
- Forensic ink analysis.
- Pharmaceutical and clinical biochemistry.
- Advantages: Simple, cheap, educational, quick.
- Limitations: Lower resolution vs TLC/HPLC, less suitable for complex mixtures.
FAQs on Paper Chromatography
1. What is paper chromatography in simple words?
Paper chromatography is a simple separation technique used to split a mixture into its parts. It uses a piece of filter paper as the base, where one liquid (the solvent) moves up the paper and carries the substances with it at different speeds.
2. What is the principle of paper chromatography?
The principle of paper chromatography is that different substances move at different speeds on paper because of how they divide between the stationary phase (water in the paper fibers) and the mobile phase (the solvent).
3. What is the stationary phase in paper chromatography?
The stationary phase in paper chromatography is the thin layer of water molecules held inside the cellulose fibers of the paper. This water helps control how far each substance moves.
4. What is the Rf value in paper chromatography?
- The Rf value in paper chromatography is a way to measure how far a substance moves compared to the solvent front. It is calculated as:
- Rf=distance of the spotdistance of the solvent frontRf = \frac{\text{distance of the spot}}{\text{distance of the solvent front}}Rf=distance of the solvent frontdistance of the spot
- It is unitless and helps in identifying compounds.
5. Why do we use a pencil and not ink in paper chromatography?
We use a pencil to mark the baseline (origin line) because pencil marks do not dissolve in the solvent. Ink would spread on the paper and interfere with the experiment results.
6. What are the applications of paper chromatography?
The applications of paper chromatography include:
- Amino acids separation in biology labs.
- Plant pigment separation such as chlorophyll and carotenoids.
- Testing food colors and preservatives.
- Forensic applications like ink analysis.
- Pharmaceutical testing for drug purity.
7. How is paper chromatography used in the food industry?
In the food industry, paper chromatography helps in food color separation and detecting artificial colorants and preservatives. This ensures the safety and quality of food products.
8. How is paper chromatography used in forensic science?
In forensic science, paper chromatography is used for ink separation in documents. It helps experts detect if a document was forged or altered by comparing different ink samples.
9. What are the advantages of paper chromatography?
The main advantages of paper chromatography are that it is cheap, simple, and easy to use. It needs only small samples and very little equipment, making it ideal for teaching and quick testing.
10. What are the limitations of paper chromatography?
The limitations of paper chromatography are that it has lower resolution compared to TLC or HPLC, and it cannot separate very complex mixtures. It is more useful for basic experiments than for advanced research.
About the Author – Gnanasree Vankadari
Gnanasree Vankadari, M.Sc. Biotechnology (Gold Medalist), is a researcher and science communicator with a strong academic background in microbiology and biotechnology. She has presented award-winning research on cryogenics and conducted a postgraduate project on Bacillus calusii. At Biology Handbook, she shares her passion for life sciences by simplifying complex biological concepts for students and readers around the world.